Abstract
Background Treatments for multiple myeloma (MM) have expanded in the last decade and the overall survival (OS) of MM patients (pts) is in continuous improvement. With the availability of new treatments and the use of high-dose chemotherapy, followed by autologous hematopoietic stem-cells transplantation (ASCT), the median OS of newly diagnosed MM (NDMM) pts, is 6-8 years. Before the introduction of ASCT and targeted therapy, the percentage of pts alive at 5 years and 10 years was 18% and 2%, respectively. To date, approximately 50% and 28% of MM patients are still alive at 5 years and 10 years. However, few data are reported in literature concerning characteristics of long term survival MM pts.
Aims The primary endpoint of this observational multicenter study is to analyze the clinical profile of MM pts who have survived 10 years or longer in order to identify possible predictors of long-term survival.
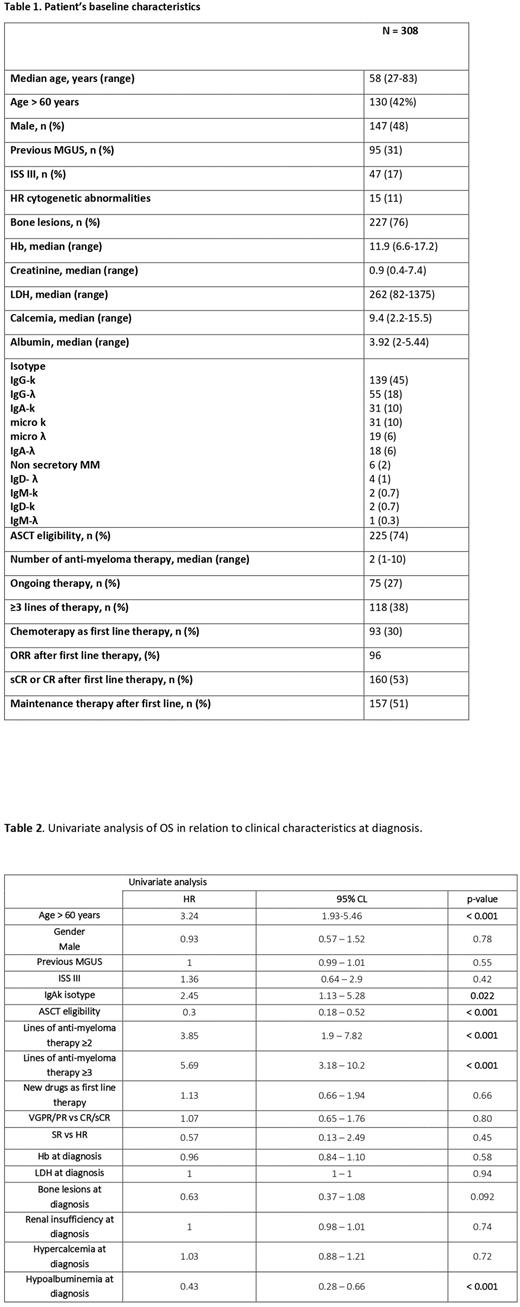
Methods and Results We retrospectively collected and analyzed data of 308 MM pts from 11 Italian hematology centers living longer than 10 years since the beginning of treatment. The clinical characteristics at diagnosis are listed in Table 1. Median age was 58 years (27-83), 130 pts (42%) were > 60 years; 161 pts (52%) were female. A history of MGUS was present in 95 pts (31%), with a median time of previous MGUS of 55 months (1-396); 196/274 pts (62%) were ISS I. The majority of pts (74%) were considered eligible for ASCT: 104 of theme (48%) had undergone a single ASCT and 112 pts (51%) a tandem ASCT. The isotype characteristics was reported in Table 1, the majority of pts showed IgG-k isotype (45%). Out of 131/308 pts evaluable for cytogenetic abnormalities, 15 pts (11%) were at high risk. The median number of anti-myeloma therapy was 2 (1-10) and the majority of pts (105) had received only one line of therapy; 118 pts (38%) had received ≥ 3 lines of therapy. At the time of data cut-off (July 2022), 67 pts (22%) were died, mostly due to progression disease and 75 pts (24%) are still in treatment and 166 (54%) are in follow-up without treatment. Ninety-three pts (30%) received conventional chemotherapy as first line therapy and 215 (70%) the new drugs. After first line, 12% of pts were in sCR; 41% of pts were in CR, 28% were in VGPR, 17% in PR and 1.6% were considered refractory to first line therapy. One hundred fifty-seven pts (51%) received a maintenance therapy after first line of therapy. Approximately, 65% of pts received second line therapy, 39% third line, 27% forth line, 15% fifth line, 9% sixth line and 4% seventh line.
After a median follow-up of 13.46 years (11.3 - 16.1), the median OS was 21.1 years (19.2-NA). The OS was 52.3% at 20 years and 45.3% at 25 years, respectively. In univariate analysis (Table 2), OS was significantly reduced in pts with: age > 60 years; not eligible for ASCT, the number of anti-myeloma therapy ≥ 3 and the IgA-k isotype. Specifically, OS at 20 years was 70% in pts with < 60 years and 10% in pts > 60 years, respectively (p<0.001). We also confirmed the role of ASCT in the prognosis of MM, independent of the induction treatment or the maintenance post - transplant therapy: pts receiving upfront ASCT have a better outcome compared to pts not eligible to ASCT (p<0.001). Considering the number of anti-myeloma therapy, there is a statistical difference in terms of OS for pts that received 1 or 2 lines of therapy and those received ≥ 3 (p< 0.001). In addition, IgA-k isotype is associated with a worse OS compared to IgG-k isotype (p=0.022). Instead, obtaining a deeper response after the first line of therapy (sCR- CR vs VGPR-PR) did not result in a statistically significant difference in terms of OS (p=0.08).
Conclusions A limited number of data are available characterizing MM pts who experienced a long survival. Despite the limit of a retrospective study, the real-life observation of our cohorts of long-term survivors with MM identified younger age, IgG-k isotype, upfront ASCT and a smaller number of anti-myeloma therapies as favorable predictors of prolonged OS. These findings underline the importance to design newly prospective studies to identify clinical, biological, and molecular characteristics that could be used to better stratify newly diagnosed MM pts in order to evaluate tailored optimal target therapies.
Disclosures
Fazio:BMS CELGENE: Honoraria; Janseen: Honoraria; gsk: Honoraria. Rossi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Morè:Janseen: Honoraria; GSK: Honoraria. Galieni:abbvie: Membership on an entity's Board of Directors or advisory committees; takeda: Membership on an entity's Board of Directors or advisory committees. Offidani:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene (Bristol Myers Squibb): Honoraria, Membership on an entity's Board of Directors or advisory committees; ROCHE: Honoraria, Membership on an entity's Board of Directors or advisory committees; JANSEEN: Honoraria, Membership on an entity's Board of Directors or advisory committees; SANOFI: Honoraria, Membership on an entity's Board of Directors or advisory committees; TAKEDA: Honoraria, Membership on an entity's Board of Directors or advisory committees. De Stefano:AbbVie: Honoraria; Amgen: Honoraria, Speakers Bureau; Bristol Myers Squibb/Celgene: Honoraria, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Petrucci:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Celgene/Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Roche: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.